Valérie Laigle - 13 Oct 2021
The COVID-19 pandemic has shone a spotlight on the need for timely access to vaccines. This is the case not only for safe and effective COVID-19 vaccines – for which we have benefitted from unparalleled speed when it comes to access and rollout – but also for other routine and novel vaccines. No-one can disagree these days that vaccination is an important component of healthcare provision and is the foremost preventive measure to protect populations against the vast consequences of the spread of infectious diseases. So how can we unlock the full value of vaccination for our societies?
In addition to maintaining robust routine immunisation programmes, another crucial factor is having the right systems in place to ensure the fast introduction of new vaccines. Combined, these two factors would give us access to not only the vaccines of today, but also the potential vaccines of tomorrow – controlling and eliminating new diseases beyond that which is currently possible. This in turn requires us to make full and proper use of the relevant tools at our disposal.
Improved access to new vaccines can only be achieved if we build on current momentum. When the median time to access to vaccination was known to be 6 years across the EU, it is clear that more needs to be done.
Vaccines Europe’s Market Access Working Group has spent the past 3 years carrying out an extensive mapping of vaccine market access pathways across all 27 EU Member States + UK and identification of key barriers and levers. The results of this exercise confirmed the complexity, heterogeneity and lack of transparency in the key steps of the vaccines market access pathways. This has now been published as a unique research-based article in the journal VACCINE, co-authored by a group of well-known experts.
The publication is filling an important gap in the knowledge of the vaccines market access pathways across all EU Member States. With the need for more transparency in this context, we are delighted to be able to share the outcomes of our research in full access, including all annexes and supplements, to a broader public, and start a reflection on urgent policy changes needed to speed up access to vaccines. It is an important step, yet we hope it is only a start, and that remaining knowledge gaps can be bridged in order to generate further reflection on appropriate actions to speed up access.
The results confirm that across Europe time to access is highly variable (see above). The time taken from marketing authorisation to population access in many countries exceeded 6 years. Only around one-third of EU countries managed to ensure access within 2 years.
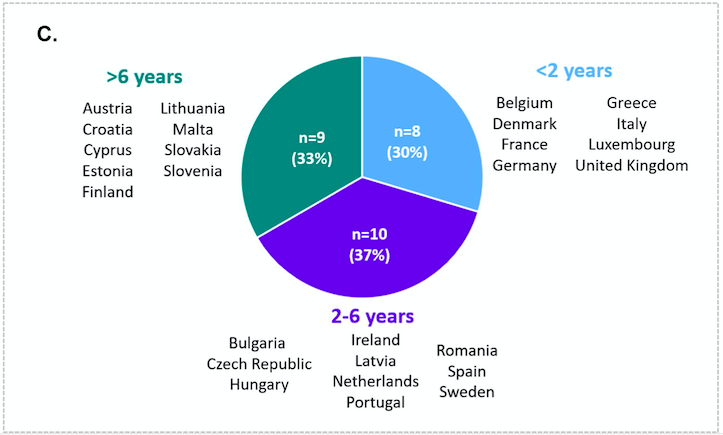
NITAG, National Immunization Technical Advisory Group; TTPA, time to population access; VMA, vaccine market access.
Main drivers to enhance Europe’s vaccination programmes include:
- Improving formal early advice,
- Removing barriers linked to complexity, heterogeneity, segmentation and limited transparency of processes,
- Developing joint clinical “fit for purpose” vaccine Health Technology Assessment (HTA)
The HTA Regulation, for which a provisional Agreement was reached just before summer this year, could be an essential tool for tackling unnecessary administrative and regulatory barriers to patients’ access to innovative medical products if it is well implemented.
Vaccines are highly complex products and unique for a variety of reasons, and therefore, their specificities need to be accounted for in HTA processes. They provide wide-ranging and positive impacts on society, but also significant economic and socioeconomic impacts – which were made clear more than ever during the COVID-19 pandemic. We cannot ignore the broad value of vaccination in the implementation stage of the HTA Regulation – in terms of processes, relevant expert-stakeholders involvement and assessment methodologies.
I hope that the sense of momentum in response to the COVID-19 pandemic will stimulate implementation of policies along the lines we recommend in the paper. Only by doing this we can improve access to vaccinations to all across the EU.
To find out more about joint clinical HTA for vaccines in Europe, you can also read Vaccines Europe’s position paper here.
Download the communication toolkit here.


 Members area
Members area

