Vaccines Europe - 12 Nov 2020
Vaccines ecosystem is very complex and challenging. In the past months, the whole world has been watching the long pathway that vaccines need to undertake – from research and development to the access to population.
Vaccines Europe has conducted research* to obtain the full picture of vaccine market access pathways in 28 EU Member States.
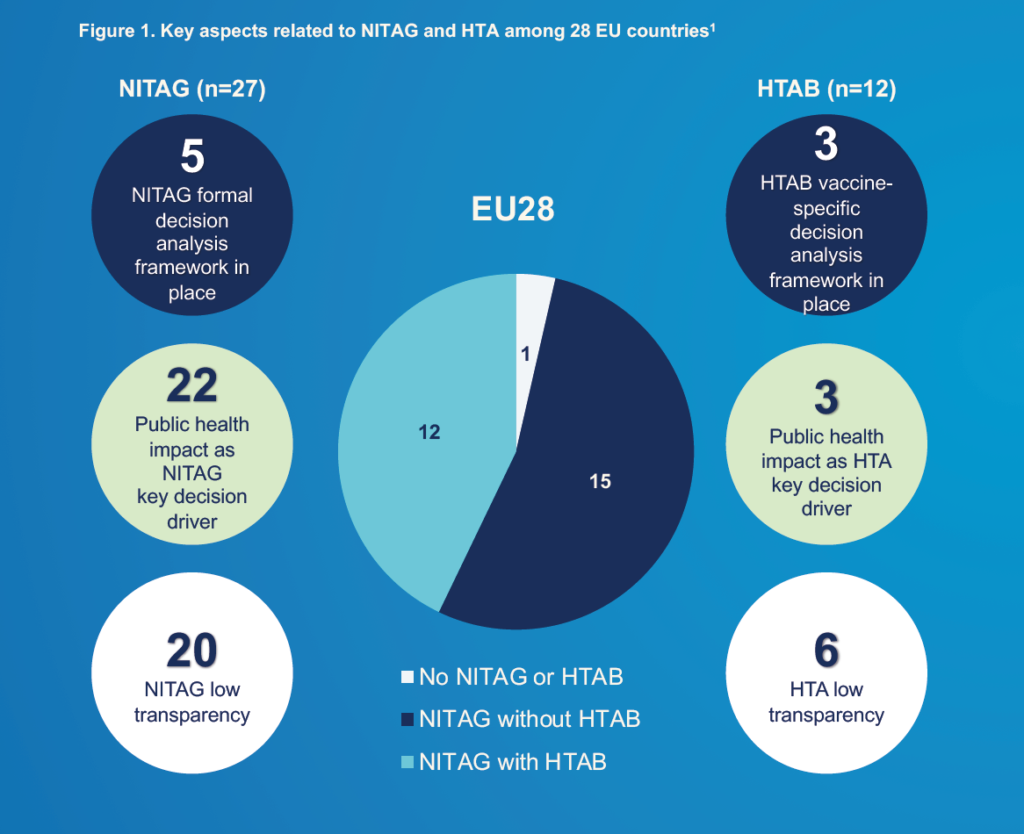
First outcomes of the project were shared during ISPOR Europe Conference 2019, mapping access decision-making process in 28 countries.
We will have a pleasure to show some additional results of the research at the upcoming Virtual ISPOR Europe Conference 2020 on 18 November 2020 as part of posters’ presentation.
What are drivers and barriers to timely access of vaccines for European populations?
Based on the interviews conducted with non-industry EU experts, it was confirmed that market access for vaccines in European member states is a highly complex, heterogeneous, lengthy and opaque decision-making process.

Disease burden (or actual benefit) has been identified as the main driver for vaccine market access. On the other hand, budget unavailability, high vaccine price, lack of cost-effectiveness and vaccination hesitancy were reported as main barriers.
Any future for EU-level collaboration on vaccines market access pathways?
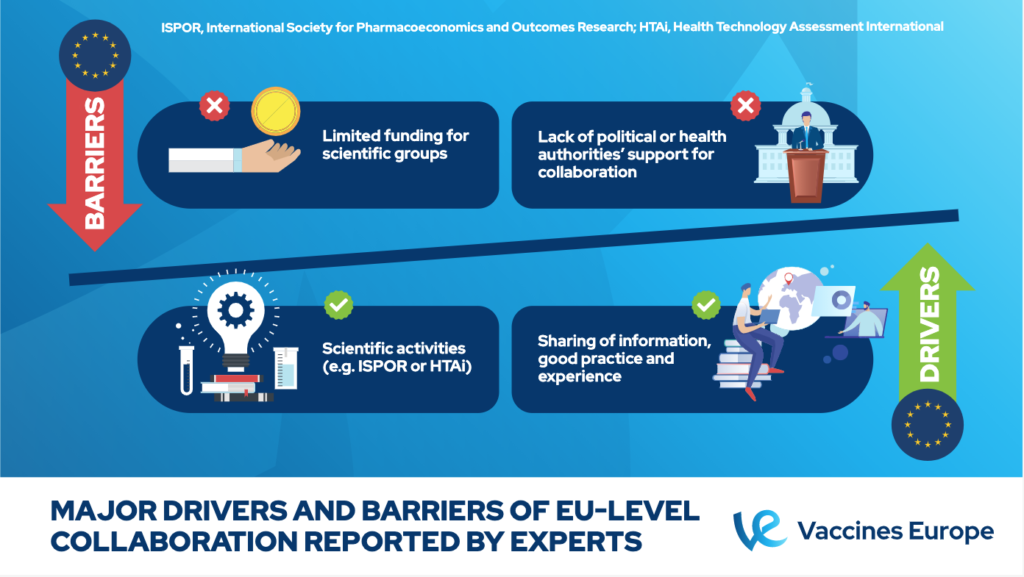
Experts identified scientific activities and sharing of information, good practice and experience as main drivers for the EU collaboration. Yet, the lack of political or health authorities’ support for such a collaboration remains a key barrier for it to happen.
Way forward – transformative period for vaccines access pathways in Europe
In the current pandemic crisis, European leaders have shown a political willingness to enhance collaboration and support fast access for pandemic vaccines in Europe. With a current momentum on rediscovering the value of vaccines, also of routine vaccines, more could be done for the future vaccines ecosystem in Europe. NITAGs timelines optimisation and implementation of Joint HTA for vaccines could be seen as one of possible trends.
***
*You may have a look at some preliminary results of our research outlined in the posters presented at the ISPOR Europe Conference 2019:

 Members area
Members area

